Abstract
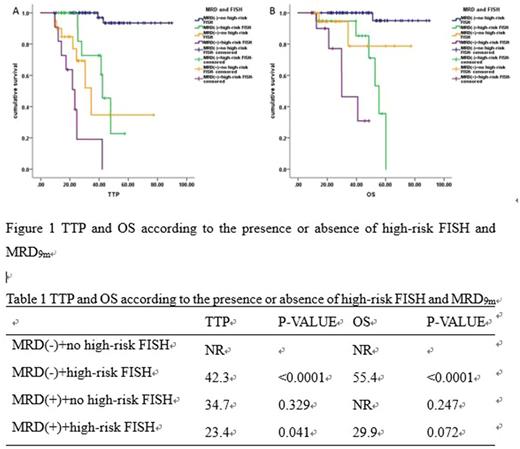
Several studies have suggested that patients with MRD-negative after autologous stem cell transplantation (ASCT) had better prognosis than MRD-positive patients, but the best timing of detection of MRD is not known. To answer these questions, we analyzed status of MRD in 141 patients with multiple myeloma who had received bortezomib induction followed with ASCT since 2010 in our center. Disease response and MRD assessment by multiparameter flow cytometry were assessed post-induction, 3 months, 6 months, 9 months and 12 months after ASCT. In order to figure out which is the best timing to assess MRD, we use MRD-negative in different time as the independent variable, time to progression (TTP) and overall survival (OS) as dependent variables, and find out the maximum area under the subject operating curve (ROC). The results suggest that MRD negative at 9 months [MRD9m] after transplantation is the best time to assess TTP (AUC=0.794, P<0.0001) and OS (AUC=0.711, P<0.0001). Further study show that baseline data, including age, ISS stage, DS stage, M protein type, level of hemoglobin, serum calcium level, LDH and serum creatinine, and high-risk FISH [17p-, t(4;14) or t(14;16)] were not associated with MRD9m. And whether or not achieving CR post- induction, post-transplantation and post-maintenance were associated with MRD9m. COX regression analysis show that the presence of high-risk FISH and MRD9m negative were independent prognosis factors for TTP and OS. In combination with high-risk FISH and MRD9m, patients with MRD9m-negative and non-high-risk FISH had best TTP and OS than those with MRD-positive or high-risk FISH, and the prognosis of MRD-positive and high-risk FISH patients were worst. Minimal residual disease positive by multiparameter flow cytometry in 9 months after autologous stem cell transplantation and high-risk cytogenetics could predict outcome in multiple myeloma receiving ASCT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal